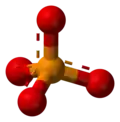
A-level Chemistry/WJEC/Module 1/Bonding
Intermolecular Forces
The bonds which act between a molecules are called intermolecular forces. The word intermolecular means, "between molecules". There are three kinds of intermolecular forces.
Van Der Waals
Temporary Dipole-Induced Dipole Also known as Induced Dipole-Induced Dipole (ID-ID) forces, London forces or Dispersion forces. These are the weakest of the intermolecular forces.
Dipole-Dipole
Hydrogen Bonding
This is the strongest type of intermolecular force. This only occurs between a lone pair of electrons on a Nitrogen (N), Oxygen (O) or Fluorine (F)atom and a hydrogen atom that has a strong partial charge(δ+). The electronegative atom pulls electrons away from the hydrogen so that, on the opposite side to the bond, the hydrogen appears almost like an unshielded proton.
Bonds
Ionic
This occurs between oppositely charged ions (atoms or molecules which have gained or lost electrons). An example of an ionically bonded compound is NaCl.
The sodium exists as a positive ion, (positively charged). It loses the electron in its sub-energy level, giving it a stable electron configuration of , or . This electron is donated into the 2p sub-energy level of the chlorine atom, which then forms a chloride ion, . This also now has a stable electron configuration of .
The force of attraction (called the "electrostatic force of attraction") between these two ions, caused by their opposite charges, is the ionic bond.
The ions arrange themselves into "giant ionic lattices", which are regular, repeating structures. Ionic compounds are usually white and crystalline in appearance; they have high melting and boiling points, as a lot of energy is required to overcome the electrostatic forces of attraction. They can, however, be disrupted by polar solvents, such as water; they are, therefore, water soluble.
Covalent
The term covalent bond is used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons. This is usually indicated as H-F. The electron pair creates a 'bond' between the two atoms because it attracts the nucleus of each atom and therefore resist the separation of the two atoms.
A co-ordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom.
Metallic
Positive ions are arranged in a lattice with a sea of delocalised (free) electrons. The force of attraction between the delocalised electrons and positively charged ions holds the structure together.
Shapes of Molecules
Methane molecule
-
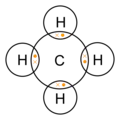
dot-cross diagram -
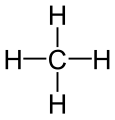
bond-line diagram -
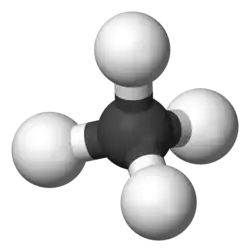
ball-and-stick model
Ammonia molecule
-
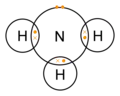
dot-cross diagram -

bond-line diagram -

ball-and-stick model with lone pairs displayed -
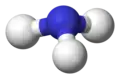
ball-and-stick model without lone pairs displayed
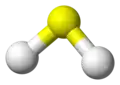
Water molecule
-
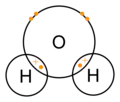
dot-cross diagram -

bond-line diagram -
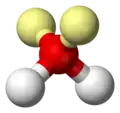
ball-and-stick model with lone pairs displayed -

ball-and-stick model without lone pairs displayed
Hydrogen fluoride molecule
-
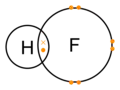
dot-cross diagram -
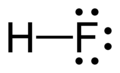
bond-line diagram -
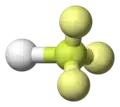
ball-and-stick model with lone pairs displayed -

ball-and-stick model without lone pairs displayed
Polyhedra
Tetrahedra
You have probably come across tetrahedra before in maths, although you most likely called them triangle-based pyramids. Tetrahedra have four vertices (corners), four faces and six edges. Each face is an equilateral triangle.
The tetrahedron is one of the most important shapes in chemistry because a very great many molecules contain them. Tetrahedral molecules don't actually contain little pyramids. What they do contain is a central atom bonded to four other atoms. The four atoms surrounding the central atom occupy positions that you can imagine as the vertices of a tetrahedron.
In the image gallery below, the central atom is coloured magenta and the surrounding atoms are coloured white.
-
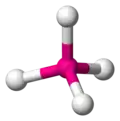
a tetrahedral molecule -
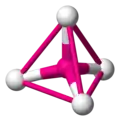
to see the tetrahedron, connect the surrounding atoms with lines -

the lines form the edges of the tetrahedron -

-

tetrahedral angle ≈ 109.5° -
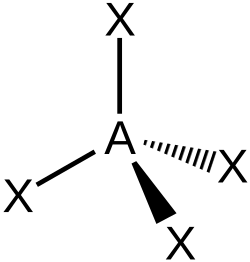
chemists represent tetrahedra using hashed and wedged bonds -
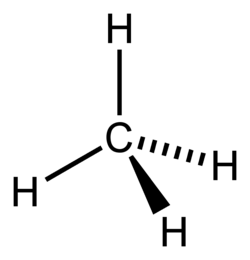
this is how chemists represent methane -

this 'flat' representation - GCSE-style - is simpler but less realistic
The angle between any two bonds in a tetrahedral molecule is approximately 109.5°. The tetrahedral angle can be calculated as accurately as required because it is equal to cos−1(–⅓).
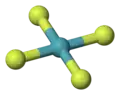
Octahedra
You may or may not have met an octahedron before. Octahedra have six vertices (corners), eight faces and twelve edges. Each face is an equilateral triangle.
Octahedra are very important in chemistry because many transition metal-based molecules are octahedral.
-

an octahedral molecule -
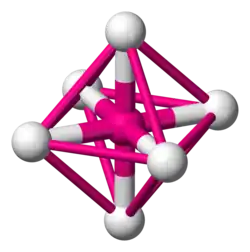
to see the octahedron, connect the surrounding atoms with lines -

the lines form the edges of the octahedron -

-
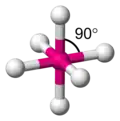
octahedral angle = 90° exactly -
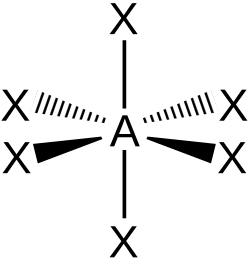
chemists represent octahedra using hashed and wedged bonds -
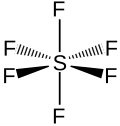
this is how chemists represent sulfur hexafluoride, SF6 -

a ball-and-stick model of SF6
Common molecular geometries
-

linear -
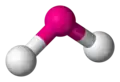
bent -
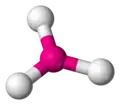
trigonal planar -
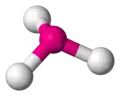
pyramidal -
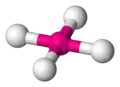
square planar -
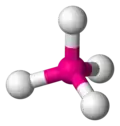
tetrahedral -
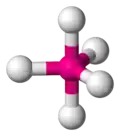
trigonal bipyramidal -
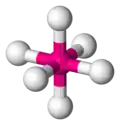
octahedral
Further examples
Example molecules
Example ions
Molecular geometry and lone pairs
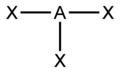
You can use the so-called AXE method to calculate the shape of a molecule. It is based on molecules that have a central atom, which we label A. Atoms or groups bonded to A are labelled X. Lone pairs are labelled E. A molecule with three lone pairs and two atoms/groups bonded to it would be denoted AX2E3. The table below shows how X and E and molecular shape are related.
Valence shell electron pair repulsion theory (VSEPR) is used to predict the shape of a molecule once X and E are known. This sounds more complicated than it is. You consider any X's and E's to be regions of charge that position themselves as far apart from each other as possible, in order to minimize the forces of electrostatic repulsion between each other.
| AXE label | X (substituents) |
E (lone pairs) |
Shape | 2D diagram lone pairs shown |
2D diagram lone pairs not shown |
3D model lone pairs shown |
3D model lone pairs not shown |
Examples |
|---|---|---|---|---|---|---|---|---|
| AX1E0 | Linear | H2 | ||||||
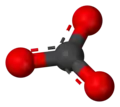
| AX2E0 | Linear | BeCl2 HgCl2 CO2 | ||||||
| AX1E1 | Linear | CN− | ||||||
| AX3E0 | Trigonal planar | 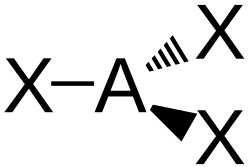
|
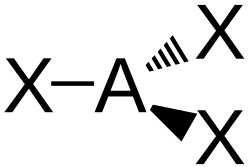
|
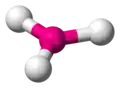
|

|
BF3 CO32− NO3− SO3 | ||
| AX2E1 | Bent | 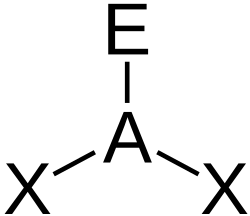
|

|
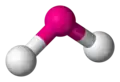
|
NO2− SO2 O3 | |||
| AX1E2 | Linear | 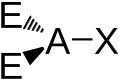
|
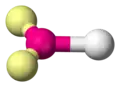
|
O2 | ||||
| AX4E0 | Tetrahedral | 
|

|
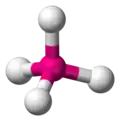
|

|
CH4 NH4+ PO43− SO42− ClO4− | ||
| AX3E1 | Trigonal pyramidal | 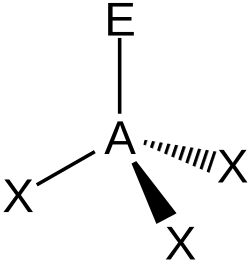
|

|

|

|
NH3 PCl3 | ||
| AX2E2 | Bent | 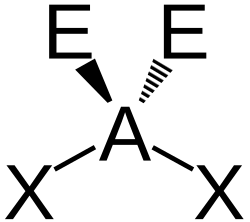
|
|
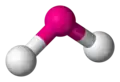
|
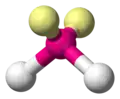
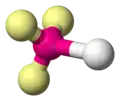
H2O H2S OF2|- | |||
| AX1E3 | Linear | 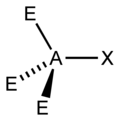
|
|
HCl | ||||
| AX5E0 | Trigonal Bipyramidal | 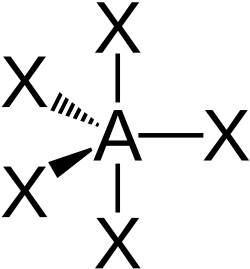
|
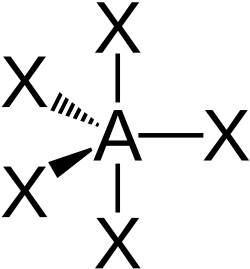
|

|

|
PCl5 | ||
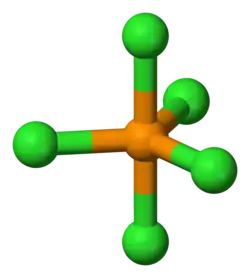
| AX4E1 | Seesaw | 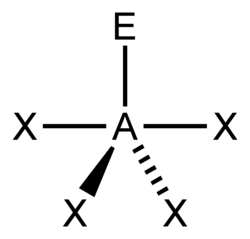
|
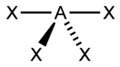
|
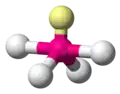
|
|
SF4 | ||
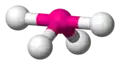
| AX3E2 | T-shaped | 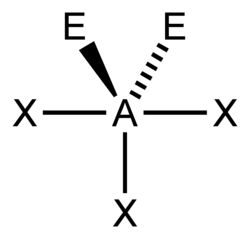
|
|
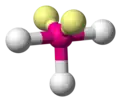
|
|
ClF3 BrF3 | ||
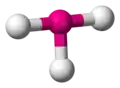
| AX2E3 | Linear | 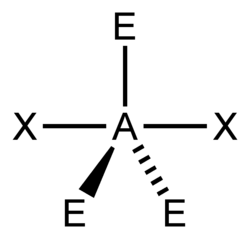
|
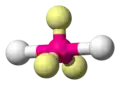
|
XeF2 I3− | ||||
| AX6E0 | Octahedral | 
|

|
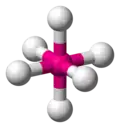
|

|
SF6 | ||
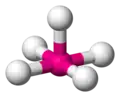
| AX5E1 | Square pyramidal | 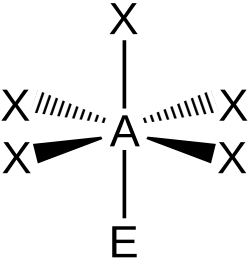
|
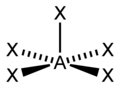
|
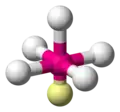
|
|
ClF5 BrF5 | ||
| AX4E2 | Square Planar | 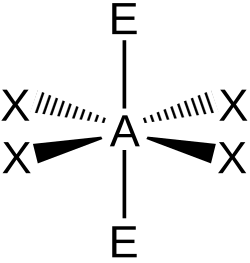
|
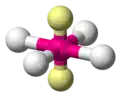
|

|
XeF4 |