A-level Chemistry/WJEC/Module 1/Calculations
The Mole
One "mole" is a number of particles. The number chosen for 1 mole is a very large, so that the mass of 1 mole of atoms, or molecules, is a convenient mass for laboratory use. e.g. 1 mole of 12C atoms have a mass of 12.0 g. Until 2020, the value of the mole was defined using 12C atoms as the standard.
The number of particles in 1 mole is the Avogadro Constant, which is given the symbol NA (or sometimes L). NA = 6.02 x 1023 mol-1 - this figure is in your Data Booklet. Since 2020 the value of NA has simply been defined as 6.022 140 76 x 1023 mol-1. The first 3 significant figures are adequate for A-level claculations.
So, a mole of particles is NA particles which is 6.02 x 1023 particles.
If a mole of 12C atoms have a mass of 12.0 g then a single 12C atom has a mass of 12.0 g ÷ 6.02 x 1023 = 1.99 x 10-23 g.
Relative Atomic Mass and Relative Molecular Mass
1 mole of the isotope 35Cl is 35.0 g. We say that the relative isotopic mass (Ir) of 35Cl is 35.0.
Chlorine naturally occurs as a mixture of two isotopes, 35Cl and 37Cl. 76 % of chlorine atoms are 35Cl. The average mass of chlorine atom is 76 % of 35 + 24 % of 37 = 26.60 + 8.88 = 35.48. This is chlorine's relative atomic mass (Ar), which we round up to 35.5.
No single chlorine atom has a mass of 35.5, the atoms have an Ir of either 35.0 or 37.0. This is similar to the average UK family having 1.5 children - some families have 1 child, some have 2 or more. On average, there are 1.5 children per family but obviously there is no such thing as 0.5 of a child.
Chlorine atoms do not usually exist on their own. Chlorine atoms usually form diatomic molecules, Cl2 which have a relative molecular mass (Mr) of 71.0. In the same way, chloroform (CHCl3) has an Mr of 119.5 (12.0 + 1.01 + 3 x 35.5).
The Ideal Gas Equation
pV=nRT
p is the pressure of the gas in Pa (Nm−2)
V is the volume of the gas in m3
- Shortcut: p is often given in kPa and V is often given in dm3.
- 22.4 dm3 at 101 kPa can be written as 0.0224 m3 at 101 000 Pa, but notice that 22.4 x 101 = 0.0224 x 101 000.
- So if p is given in kPa and V is given in dm3 then you need not convert p to Pa and V to m3 - just multiply the values you're given.
n is the amount (number of moles)
R is the gas constant (8.31 J K−1 mol−1)
T is the temperature in kelvin (equivalent to 273 + θ, the temperature in degrees Celsius)
Empirical and Molecular Formulae
Definitions:
- Empirical formula: The simplest whole number ratio of atom types present in a molecule of a compound.
- Molecular formula: The actual number of atoms of each element present in a molecule of a compound.
To determine a molecular formula you need the given mass of the substance and the empirical formula mass. Divide the given mass by the empirical formula mass.
Multiply the given answer by the ratios in the empirical formula and you will have the molecular formula.
Balanced Equations and Associated Calculations
Errors
It is important to know how certain a measurement is; What is the potential error in the measurement?
A 25 cm3 volumetric pipette is marked ± 0.06 cm3. This is the absolute uncertainty, u.
If the uncertainty is not recorded, we can assume that the absolute uncertainty is:
- one-half of the divisions drawn on a scale
- one-half of the last decimal place shown on a digital display
For a ruler which has a 1 mm scale, the absolute uncertainty is ± 0.5 mm. For a digital thermometer showing 24.2 ᐤC, the absolute uncertainty is ± 0.05 ᐤC.
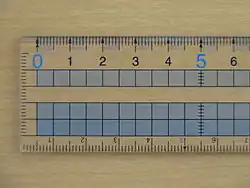

Be careful, many measurements are actually the difference between two measurements. If we used the rule to measure 297 mm, we rely on the 0 mm and the 297 mm marks being accurate. If we add the two absolute uncertainties we find that we have measured 297 mm ± 1 mm†. This is true of burettes and balances.
† To be exact, we shouldn't add the uncertainties, but square the uncertainties, add the squares, and then take the square root: √(0.52 + 0.52) = 0.71 mm. There's no time for this at A-level: Just add the uncertainties!
Examples:
- Initial burette reading: 0.4 cm3. The scale is marked every 0.1 cm3 so this initial reading is 0.4 ± 0.05 cm3.
- Final burette reading: 24.7 cm3 ± 0.05 cm3.
- Titre: 24.7 - 0.4 = 24.3 ± 0.1 cm3
- Initial burette reading: 0.0 ± 0.05 cm3.
- Final burette reading: 24.3 cm3 ± 0.05 cm3.
- Titre: 24.3 - 0.0 = 24.3 ± 0.1 cm3
This is why it is often a waste of time to "zero" a burette. Just record the initial and final values.
- Mass of weighing boat: 1.210 g. The digital scale gives three decimal places so this reading is 1.210 ± 0.0005 g.
- Mass of weighing boat with sample: 2.210 ± 0.0005 g.
- Mass of weighing boat after sample transferred to beaker: 1.211 ± 0.0005 g.
- Mass of sample transferred to beaker: 2.210 - 1.211 = 0.999 ± 0.001 g
Notice how it is important to record the final zero if you have measured it. 0.0 cm3 is more precise than 0 cm3. 1.210 g is more precise than 1.21 g.
What if you use the 0.999 g of sample to make a 250 cm3 solution, and measure 24.3 cm3 from this solution using the burette? How many grams of sample are in the 24.3 cm3?
Well, 0.999 g x 24.3 cm3 ÷ 250 cm3 = 0.0971 g but how certain is this value?
We have three uncertainties to consider:
- Balance: 0.999 ± 0.001 g
- Volumetric flask: 250 ± 0.3 cm3
- Burette: 24.3 ± 0.1 cm3
The uncertainties add up, but how?
We can calculate the relative (percentage) uncertainty, ur from the equation ur = 100 % x absolute error ÷ the value measured.
- Balance: 100 % x 0.001 g ÷ 0.999 g = 0.100 %
- Volumetric flask: 100 % x 0.3 cm3 ÷ 250 cm3 = 0.120 %
- Burette: 100 % x 0.1 cm3 ÷ 24.3 cm3 = 0.411 %
Adding the percentage errors gives us ± 0.631 % (or 0.440 % using the square-root-of-the-sum-of-the-squares method you really don't need to know).
The 24.3 cm3 of solution contains 0.0971 g ± 0.631 %.
Significant figures
As a rule, give your answers to 3 significant figures unless told otherwise. This implies a percentage error between 0.5 % and 0.05 %.
- 0.999 g could be between 0.9995 and 0.9985 g so ± 0.0005 g or ± 0.05 %
- 0.100 g could be between 0.1005 and 0.0995 g so ± 0.0005 g or ± 0.5 %
- 2 significant figures implies a percentage error between 5 % and 0.5 %.
- 3 significant figures implies a percentage error between 0.5 % and 0.05 %.
- 4 significant figures implies a percentage error between 0.05 % and 0.005 %.
If you use several values in a calculation, the value with the lowest number of significant figures dictates the number of significant figures in the answer.
Example: 0.998 g ÷ 2.0 dm3 = 0.50 g dm-3
Exception: When adding or subtracting numbers, the value with the lowest number of decimal places dictates the number of decimal places in the answer.
Example: 2.0 g - 0.998 g = 1.0 g