A-level Chemistry/WJEC/Module 1/Solids
Introduction
If cold enough, all substances+ will freeze into solids. Some force must hold their atoms together, even if it's a very weak force that only becomes important at very low temperature.
When a substance melts, it is important to think about which bonds/forces break. Substances with covalent bonds usually also have intermolecular forces such as:
- induced dipole-induced dipole (London) forces
- dipole-dipole forces
- hydrogen bonds
Covalent bonds are stronger than these intermolecular forces, so when covalent materials melt it is usually the weak intermolecular bonds which break, and the covalent bonds remain intact.
+Except helium, which is weird stuff.
Examples of solid structures
WJEC give these examples in pairs, and they are good to analyse with a "compare and contrast" method:
Sodium chloride and caesium chloride


Compare: These two compounds are ionic structures. In both cases, the chloride anion is attracted to the metal cation and an ionic lattice forms. The bonding is strong and the melting points are high (1074 K for NaCl, 919 K for CsCl).
Contrast: The big difference is that the caesium ion is larger than the sodium ion. Each caesium can hold eight chloride ions around it, without the chloride ions getting so close to one another that they start repelling each other. Sodium ions are smaller, and only six chlorides can surround each sodium ion.
There are many ionic structures where the cation is large enough to form a CsCl-type lattice (with a coordination number of eight), but other factors mean an NaCl-type lattice (six-coordinate) forms instead.
Diamond and graphite

Compare: These two forms of pure carbon ("allotropes") have covalent bonding between carbon atoms. The bonding is totally non-polar. Both diamond and graphite have very high melting points (4600 K is often quoted, but melting these materials is complicated), which means that the melting process involves breaking strong bonds.
Contrast: In diamond, there are only covalent bonds. The bonds are all single bonds and a giant lattice is formed, with each carbon bonded to four other carbons in a tetrahedral arrangement. The strong covalent bonds cause the high melting point. The strong lattice makes diamond the hardest known material. The electrons in a single bond are not free to move, so diamond does not conduct electricity. The lattice is efficient at passing on vibrations, so diamond is a good conductor of heat.
In graphite the carbon atoms form sheets with alternating single and double covalent bonds arranged in a hexagonal pattern. The electrons in the bonds can swap positions so graphite will conduct electricity. Electrical conductivity is much less if measured at right-angles to the graphite sheets, because electrons are not shared between one sheet and another. Most graphite samples are a jumble of small, disordered sheets so this directional ("anisotropic") effect is not normally important.
The graphite sheets are held together by ID-ID forces. These forces do not depend on the positions of the atoms they hold together, which allows the sheets to slide past one another. Graphite is slippery and can be used as a solid lubricant. ID-ID bonds are weak, but the massive surface area of graphite sheets means the ID-ID forces accumulate and bond the sheet together very strongly, which explains the high melting point.
Heat is easily conducted through each graphite sheet by its covalent bonds. Thermal conductivity is many times less efficient from one sheet to the next because of the weaker ID-ID bonds between sheets. As with electrical conductivity, the anisotropic differences are not usually significant.
Iodine and ice
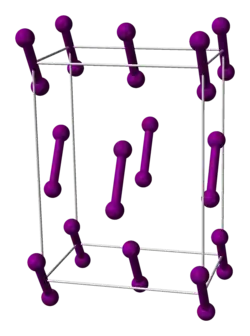
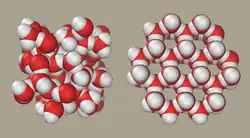
Compare: Iodine (I2(s)) and ice (H2O(s)) are both small molecules with atoms bonded by single covalent bonds. In the solid form, the molecules are held together by intermolecular forces. Melting involves the breaking of intermolecular forces, not the covalent bonds. The low melting points of ice and iodine show how weak the intermolecular forces are.
Contrast: Iodine is a simple diatomic, non-polar molecule. The only intermolecular force is the ID-ID force. It is easy to break this weak force and melt iodine; It melts at 387 K. The solid structure has a "herring-bone" pattern.
Water molecules are angular, polar molecules with a partial negative charge on the oxygen atom. Water molecules form hydrogen bonds very efficiently; Each H2O molecule can both accept and donate two hydrogen bonds. Hydrogen bonds, in general, are the strongest type of intermolecular bond. The melting point of ice is 273 K.
It is easier to melt ice, with its hydrogen bonds, than it is to melt iodine by breaking its ID-ID forces. Hydrogen bonds are supposed to be stronger? Remember that I2 is a much larger molecule than H2O, so the ID-ID forces are strengthened by iodine's large surface area and many electrons.
In ice, the hydrogen bonds need to be arranged tetrahedrally around each oxygen atom. In water, the molecules can come closer together because they do not have to form a tetrahedral lattice. This is why ice is less dense than water.
Metallic bonding
Metallic bonding is the final piece of the bonding puzzle;
- Two non-metal atoms bond by covalent bonding.
- A metal and a non-metal atom bond by ionic bonding.
- Two metal atoms bond by metallic bonding.
Metal atoms are more stable if they release electrons and become cations. In metallic bonding, where the only atoms are metal atoms, there is nothing to accept the electrons, and so the electrons form a fluid "sea" of mobile electrons.
The mobility of the electrons allows them to carry charge across the metallic structure. Metals conduct electricity. Metals conduct heat well because the mobile electrons can transfer thermal energy through the structure. Thermal conductivity in metals is approximately proportional to their electrical conductivity. The electrons absorb and re-emit light at all wavelengths, which makes metals reflective. Most metals appear "silvery" i.e. they have no colour of their own. Only two metals have significant colour.
-
 A bar of copper.
A bar of copper. -
 A bar of silver. Most metals look similar to silver.
A bar of silver. Most metals look similar to silver. -
Bars of gold.
-
 Liquid mercury.
Liquid mercury.
If the metal cations move into new positions, the electrons simply move with them and the bonding is maintained. Metals are ductile and malleable - their rigid shapes can be altered without breaking the structure.
Metallic bonding is strong, so the melting points are generally high. The lowest melting point of a metal is mercury at 234 K and the highest is tungsten at 3695 K. The highest melting points occur in metals where the atoms release multiple electrons and/or form small cations.
Physical properties
Physical properties include:
- Boiling point
- Melting point
- Electrical conductivity
- Thermal conductivity
- Hardness
- Brittleness
- Solubility in water
| Property | Metallic | Ionic | ID-ID only+ | Covalent only | Covalent plus intermolecular |
|---|---|---|---|---|---|
| Melting and boiling points | High | High | Low | High | Low, but depends in the type of intermolecular bond and the size of the covalent molecule |
| Electrical conductivity | High | None in solid state, but conductive if molten or in solution. | None | None | None, unless electrons are delocalised e.g. graphite |
| Thermal conductivity | High, due to mobile electrons | Fairly high, due to rigid nano-structure. | None | Fairly high, due to rigid nano-structure. Diamond is a very good conductor of heat. | None, unless there are large, rigid molecules |
| Hardness | High | High | Low | High | Low, unless there are large, rigid molecules |
| Brittleness | Low - metals are malleable and ductile | High | Low | High | Low, unless there are large, rigid molecules |
| Solubility in water | None, but many metals react with water | Often soluble, if ionic lattice is weaker than the water-ion bonding | Low | None, but many covalent substances react with water | Low for large molecules, and for molecules which only ID-ID bond. Small molecules with dipole-dipole bonding or hydrogen bonding are very soluble. |
+Noble gases are the only substances that form structures with only ID-ID bonding.