A-level Chemistry/WJEC/Module 2/Halogenoalkanes
Halogenoalkanes are otherwise simple alkanes that contain one or more members of the halogen family. In practice, the halogens found in organic molecules are chlorine (Cl), bromine (Br), fluorine (F), and iodine (I). Some texts refer to this class of compounds as haloalkanes or alkyl halides. The chemical literature will frequently use the terms interchangeably.
Note: The X in R-X represents a generic halogen atom.
Naming Halogenoalkanes
Haloalkanes are named by adding a prefix to the name of the alkane from which they are derived. The prefix denotes the particular halogen used.
F = Fluoro-
Cl = Chloro-
Br = Bromo-
I = Iodo-
If other substituents need to be named, all prefixes are still put in alphabetical order. When necessary, numbers identify substituent locations.
Example names of halogenoalkanes
| IUPAC name | Common name | |
| CH3—F | FluoroMethane | Methyl fluoride |
| CH3—Cl | ChloroMethane | Methyl chloride |
| CH3—Br | BromoMethane | Methyl bromide |
| CH3—I | IodoMethane | Methyliodide |
| F—CH2—F | DiFluoroMethane | Methylene fluoride |
| Cl—CH2—Cl | DiChloroMethane | Methylene chloride |
| F—CH2—Cl | ChloroFluoroMethane | |
| CHBrClF | BromoChloroFluoroMethane | |
| HCCl3 | TriChloroMethane | Chloroform |
| CHX3 | Haloforms (X=halogen) | |
| CCl4 | TetraChloroMethane | Carbon tetrachloride |
| CH3CHCl2 | 1,1-DiChloroEthane | |
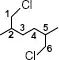
|
1,6-DiChloro-2,5-DiMethylHexane |
Physical properties
R-X bond polarity: C—F > C—Cl > C—Br > C—I
| atom | | electronegativity | | difference from C (= 2.5) | |
| F | 4.0 | 1.5 |
| Cl | 3.0 | 0.5 |
| Br | 2.8 | 0.3 |
| I | 2.5 | 0.0 |
The difference in electronegativity of the carbon-halogen bonds range from 1.5 in C-F to almost 0 in C-I. This means that the C-F bond is extremely polar, though not ionic, and the C-I bond is almost nonpolar.
Physical appearance: Halogenoalkanes are colourless when pure. However bromo and iodo alkanes develop colour when exposed to light. Many volatile halogen compounds have sweet smell.
Boiling point: Halogenoalkanes are generally liquids at room temperature. Halogenoalkanes generally have a boiling point that is higher than the alkane they are derived from. This is due to the increased molecular weight due to the large halogen atoms and the increased intermolecular forces due to the polar bonds, and the increasing polarisabilty of the halogen.
For the same alkyl group, the boiling point of halogenoalkanes decreases in the order RI > RBr > RCl > RF. This is due to the increase in van der Waals forces when the size and mass of the halogen atom increases.
For isomeric halogenoalkanes, the boiling point decreases with increase in branching.
Density: Halogenoalkanes are generally more dense than the alkane they are derived from and usually more dense than water. Density increases with the number of carbon and halogen atom. It also increases with the increase in mass of halogen atom.
Solubility: The halogenoalkanes are only very slightly soluble in water, but dissolve in organic solvents. This is because for dissolving halogenoalkanes in water the strong hydrogen bonds present in water have to be broken. When dissolved in organic (non polar) solvents, the intermolecular attractions are almost same as that being broken.
Bond Length: C—F < C—Cl < C—Br < C—I
| bond | length (pm) |
| C-F | 138 |
| C-Cl | 177 |
| C-Br | 193 |
| C-I | 214 |
Larger atoms means larger bond lengths, as the orbitals on the halogen is larger the heavier the halogen is. In F, the orbitals used to make the bonds is 2s and 2p, in Cl, it's 3s and 3p, in Br, 4s and 4p, and in I, 5s and 5p. The larger the principal quantum number, the bigger the orbital. This is somewhat offset by the larger effective nuclear charge, but not enough to reverse the order.
Chemical properties
Bond strength: C—F > C—Cl > C—Br > C—I
| bond | strength (kJ mol-1) |
| C-F | 484 |
| C-Cl | 338 |
| C-Br | 276 |
| C-I | 238 |
The orbitals C uses to make bonds are 2s and 2p. The overlap integral is larger the closer the principal quantum number of the orbitals is, so the overlap is larger in the bonds to lighter halogens, making the bond formation energetically favorable.
Bond reactivity: C—F < C—Cl < C—Br < C—I
Stronger bonds are more difficult to break, making them less reactive. In addition, the reactivity can also be determined by the stability of the corresponding anion formed in solution. One of the many trends on the periodic table states that the largest atoms are located on the bottom right corner, implying that iodine is the largest and fluorine being the smallest. When fluorine leaves as fluoride (if it does) in the reaction, it is not so stable compared to iodide. Because there are no resonance forms and inductive stabilising effects on these individual atoms, the atoms must utilise their own inherent abilities to stabilise themselves. Iodide has the greatest surface area out of these four elements, which gives it the ability to better distribute its negative charge that it has obtained. Fluorine, having the least surface area, is much more difficult to stabilize. This is the reason why iodine is the best leaving group out of the four halogens discussed.
Reactions
Substitution reactions of halogenoalkanes
R-X bonds are very commonly used throughout organic chemistry because their polar bonds make them reasonably reactive. In a substitution reaction, the halogen (X) is replaced by another substituent (Y). The alkyl group (R) is not changed.
|
The ":" in a chemical equation represents a pair of unbound electrons. |
|
A general substitution reaction Y: + R—X → R—Y + X: |
Substitutions involving halogenoalkanes involve a type of substitution called Nucleophilic substitution, in which the substituent Y is a nucleophile. A nucleophile is an electron pair donor. The nucleophile replaces the halogen, which becomes a leaving group. Nucleophilic substitution reactions are abbreviated as SN reactions.
|
"Nu" represents a generic nucleophile. |
General nucleophilic substitution reactionsNu:- + R—X → R—Nu + X:- |
| Reagent | Nucleophile | Name | Product | Product name |
| NaOH/KOH | :O-H | Hydroxide | R—OH | Alcohol |
| NaOR' | :O-R' | Alkoxide | R—O—R' | Ether |
| :S-H | Hydrosulfide | R—SH | Thiol | |
| NH3 | :NH3 | Ammonia | R—NH3+ | Alkylammonium ion |
| KCN | :C-N | Cyanide | R—CN | Nitrile |
| AgCN | Ag-CN: | Silver cyanide | R-NC | isonitrile |
| :C-≡C—H | Acetylide | R-C≡C—H | Alkyne | |
| NaI | :I- | Iodide | R—I | Alkyl Iodide |
| R'-M+ | :R'- | Carbanion | R-R' | Alkane |
| KNO2 | O=N—O | Nitrite | R–O—N=O | Alkyl nitrite |
| AgNO2 | Ag—Ö—N=O | Silver nitrite | R—NO2 | Nitroalkane |
| LiAlH4 | H | Hydrogen | RH | alkane |
| R'COOAg | R'COO- | Alkanoate | R'COOR | Ester |
Example: Suggest a reaction to produce the following molecule.
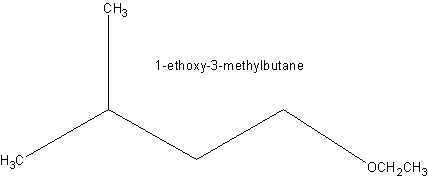
Answer:
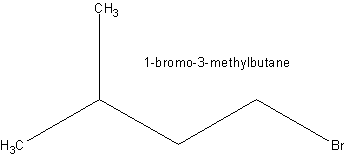 +
+![]()
OR
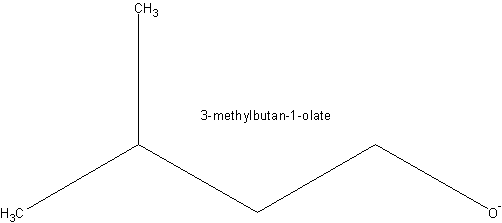 +
+![]()
Any halogen could be used instead of Br
Reaction mechanisms
Nucleophilic substitution can occur in two different ways, but we only consider the SN2 mechanism.

Elimination reactions
With alcoholic potassium hydroxide, halogenoalkanes lose H-X and form the corresponding alkene.