A-level Chemistry/WJEC/Module 2/Organic
Displaying Molecular Formulae
There are several methods for displaying molecular structures. The most detailed is a displayed structure:
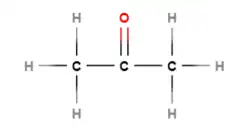
All the atoms and all the bonds are shown.
A skeletal formula reduces the structure to a simple outline.
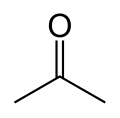
Hydrogen atoms bonded to carbon atoms are not shown, not even their bonds. Carbon atoms are not shown, but bonds between carbon atoms show where they must be located.
A shortened formula gives each carbon atom in turn, but does not include bonds.
Propanone's shortened formula is CH3COCH3.
The molecular formula simply lists the atoms in a molecule.
Propanone's molecular formula is C3H6O.
The empirical formula only gives the proportions of the elements in a molecule.
Propanone's empirical formula is the same as its molecular formula: C3H6O.
Lactic acid:

Lactic acid's shortened formula is CH3CHOHCOOH. The molecular formula is C3H6O3 and the empirical formula is CH2O.
Naming Organic Molecules
NB: It is helpful to add Capital Letters to long scientific words. It helps to see how the word is made up. Example: ChloroFluoroCarbon. This unusual format is known as "CamelCase".
Rules for naming organic molecules:
- Find the longest carbon chain and the alkane it corresponds to (methane, ethane, propane, butane, pentane, etc).
| Number of carbon atoms | Prefix | Number of carbon atoms | Prefix |
|---|---|---|---|
| 1 | meth- | 6 | hex- |
| 2 | eth- | 7 | hept- |
| 3 | prop- | 8 | oct- |
| 4 | but- | 9 | non- |
| 5 | pent- | 10 | dec- |
Take care here! A compound should be displayed with the longest chain written horizontally, but in an exam this is not the case. At first sight, the molecule might be seen to have a longest chain from one side to another, but in fact the longest straight chain incorporates carbon atoms that are above or below the horizontal, so the chain is longer than you think.
- Any branching chains are named using the same system: methyl, ethyl, propyl, etc.
- Use numbers if necessary to say where the branch is attached e.g. 2-MethylPentane and 3-MethylPentane are isomers. 4-MethylPentane is the same as 2-MethylPentane with the atoms counted from the other end of the pentane chain. Use the lower number.
- Use “di”, “tri”, “tetra” etc if several identical branches are attached e.g. DiMethylPropane, TetraMethylButane. Add numbers if necessary e.g the isomers 2,2-DiMethylPentane, 2,3 DiMethylPentane, 2,4-DiMethylPentane and 3,3-DiMethylPentane are isomers of EthylPentane.
- When incorporating the branch names, keep them in alphabetical order, i.e., ethyl comes before methyl.

- Halogen atoms are named like methyl branches e.g. 1,1,1-TriChloroEthane, DiChloroDiFluoroMethane

- Alkenes (with a C=C bond) alter the end of the alkane name. Similar rules for multiple C=C bonds and positions apply: e.g. Ethene, Propene, But-1-Ene (CH2=CHCH2CH3), Buta-1,3-DiEne (CH2=CHCH=CH2).
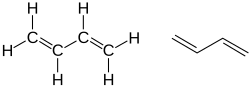
- Carboxylic acids alter the ending too: Ethanoic Acid, Butanoic Acid, etc. The -COOH group can only be at the end of a carbon chain so numbers are rarely needed. HOOC-COOH is EthaneDioic Acid, HOOC CH=CHCOOH is ButeneDioic Acid.
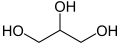
- Alcohols add "-ol" to the end of the name, or sometimes "hydroxy-" to the beginning if another ending is required. E.g. Ethanol, Propan-1-ol, Propane-1,2,3-Triol, 2-HydroxyPropanoic Acid
Example: Propane-1,2,3-Triol (Glycerol); CH2OHCHOHCH2OH (shortened), C3H8O3 (molecular/empirical).
-
 Displayed formula
Displayed formula -
 Skeletal formula
Skeletal formula
Isomers
Definition: Isomers are different chemical compounds which have the same molecular formula. (Do not confuse isomers (different molecules) with isotopes, which are different atoms. Isotopes are atoms with different mass numbers which have the same atomic number.
C4H10 has two isomers; Butane CH3CH2CH2CH3 and MethylPropane CH3CH(CH3)CH3
-
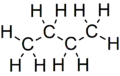 Butane
Butane -
 MethylPropane
MethylPropane
These are a type of structural isomer called chain isomers. Structural isomers have different patterns of bonds ("conformations"). Chain isomers have different arrangements of the main carbon chain.
C3H8O has three isomers; Propan-1-ol, CH3CH2CH2OH, Propan-2-ol, CH3CHOHCH3, and MethoxyEthane, CH3CH2OCH3.
-
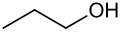 Propan-1-ol
Propan-1-ol -
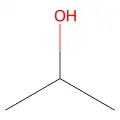 Propan-2-ol
Propan-2-ol -
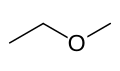 MethoxyEthane
MethoxyEthane
These three are also structural isomers. Propan-1-ol and propan-2-ol are positional isomers; The difference is where the -OH group is attached. MethoxyEthane (not a type of compound you need to know) is not an alcohol, which makes it a functional group isomer of the other two isomers.