A-level Chemistry/WJEC/Module 2/Rates
Chemical kinetics is the study of the rates of chemical reactions. You may know if a reaction is capable of happening, and you may know how far the reaction will proceed, but you don't know fast it will happen. Consider two reactions: the rusting of an iron nail and the combustion of propane. Both reactions will occur, and both will occur to completion. The rusting will take years to complete, but propane will combust in an instant. Furthermore, the nail will rust faster when it is moist, and slower in the presence of less oxygen. Obviously, there are factors that affect the rates of chemical reactions. The study of these factors and rates is chemical kinetics.
-
 This iron wire has taken years to become rusty.
This iron wire has taken years to become rusty. -
This fire took only a moment to start.
Reaction Rate
| Consider this generic chemical reaction. (Lower case letters represent the molar coefficients.) | |
| The reaction rate is defined as the rate of change of the concentration of the substances. Remember that a substance written inside brackets is its concentration. The reaction rate written this way involves calculus, but in non-mathematical terms it is simply the rate of change of the concentrations. |
In summary, the reaction rate can be measured by monitoring the decreasing concentration of a reactant, or the increasing concentration of a product. The reaction rate measures the speed at which a reaction proceeds.
In practical terms, v = change of chemical ÷ time taken for the change
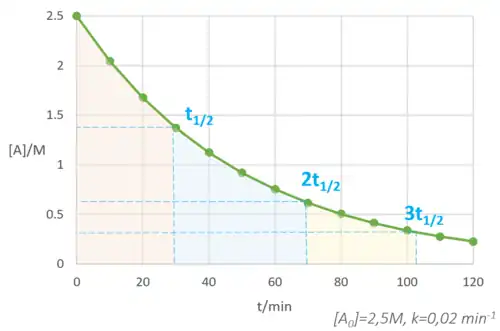
On a graph of concentration vs time, the rate of the reaction is represented by the gradient of the graph. In the graph above, the rate (gradient) is not constant - that would be a straight line - so how do we measure the rate? We need to draw a tangent to the curve at the point we want to measure its gradient.
At the beginning of a reaction, we can get away with the "initial rate assumption" - if we measure the rate quickly, we can assume that (1) the concentrations have not changed significantly (even if we do measure the concentration changes to measure the rate!) and (2) the rate is constant i.e. on a graph we are looking at such a small part of the curve that we can treat it as a straight line.
In the example, the concentration falls from 2.50 to 2.05 mol dm-3 in 10 minutes. This means we can estimate the rate is 0.045 mol dm-3 min-1 with the initial rate assumption.
Using a tangent to the graph, we would find the actual initial rate is 0.050 mol dm-3 min-1, so the initial rate assumption caused a 10 % error. This error might be acceptable, especially if we only need to compare reaction rates and the error is consistent.
In many cases, we set up experiments where the reaction reaches a specific point after a time:
- The reaction mixture becomes so cloudy that a cross at the bottom of the container is no longer visible
- Enough iodine is produced to turn the solution dark blue/black
We usually reckon, not only that;
- v = change of chemical ÷ time taken for the change
But, because we designed the chemical change to be the same for each experiment;
- v ∝ 1 ÷ time taken for the change (v is proportional to the time taken for the change)
The clock reaction in the above video gives these results
| Volume of peroxide (cm3) | Time taken (s) | v ∝ 1/time (s-1) |
|---|---|---|
| 10 | 52 | 0.019 |
| 15 | 35 | 0.029 |
| 20 | 26 | 0.038 |
| 25 | 22 | 0.045 |
| 30 | 18 | 0.056 |
Notice how the volume of peroxide is proportional to the 1/time value. Not only are we using 1/time as a value that is proportional to the reaction rate, but we are also using "volume of peroxide" to mean roughly "concentration of peroxide" - if the same concentration of stock peroxide is used in the mixture, and the total mixture volume is the same, then the volume will be proportional to the concentration. If it works, why make it more complicated?
Collision Theory
Collision theory predicts that reactions occur when molecules collide. In order for reactants to form products, the reactant molecules must physically collide so that they can rearrange themselves into product molecules. Only some collisions are effective because the collision must involve enough energy to allow the reaction to occur. This is called activation energy, the energy needed to begin a reaction.
Activation energy explains why petrol will not spontaneously ignite. First, a small spark or flame must be present. The heat generated by the spark gives the petrol molecules (a mixture of small hydrocarbon molecules) enough energy to activate the reaction. Being highly exothermic, the combustion of petrol releases a large amount of heat — more than enough to activate further reactions and create a fire.
Factors Affecting Rate
The rate of a reaction is affected by many factors. These effects can be measured empirically or explained by collision theory.
Concentration
Increasing the concentration of the reactants will (almost always) increase the rate they react.
Collision theory explains this. Higher concentrations means more molecules packed into a given space. Therefore, there will be more collisions. More collisions mean more effective collisions (and also more ineffective collisions, but who cares about them?) and therefore products will form faster.
Pressure
In a reaction of gaseous reactants, the partial pressure of the gases has the same function as the concentration.
However, increasing the overall pressure (or decreasing the volume if you remember the gas laws) will also result in a greater reaction rate. The increased pressure causes the molecules to collide more frequently. More collisions mean that products will form faster.
Temperature
As you should already know, a molecule's kinetic energy is directly proportional to its temperature. By increasing the temperature, molecules collide more vigorously, and more collisions will be effective.
Surface Area and Stirring
In a heterogeneous reaction there are two or more phases of matter interacting, such as a solid dissolving into a liquid, or two immiscible liquids such as oil and water. The reaction can only occur at the interface between the two phases.
Increasing the surface area of the interface will increase the reaction rate. This might be by braking up a solid reactant into smaller pieces, or by emulsifying an oily liquid into tiny droplets so it can react with an aqueous solution.
The concentrations at the interface will change rapidly - reactants are depleted and products accumulate. There might still be plenty of reactant in the rest of the mixture, so stirring or shaking the mixture will speed up the reaction rate.
Catalysts
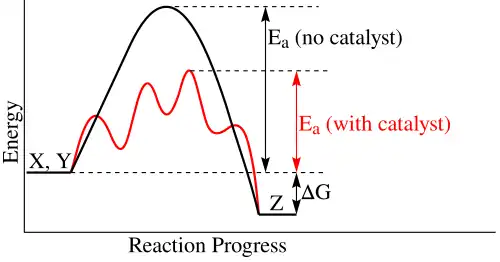

A catalyst is a substance that helps a reaction proceed without being consumed. A catalyst provides a reaction pathway with lower activation energy.
In biochemistry, an enzyme is a protein that serves as a catalyst.