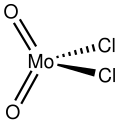
Molybdenum dichloride dioxide
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.480 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| MoO2Cl2 | |
| Molar mass | 198.85 g·mol−1 |
| Appearance | yellow or cream solid |
| Melting point | 175 °C (347 °F; 448 K) |
| Hazards | |
| GHS labelling:[1] | |
| |
| Danger | |
| H314 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Molybdenum dichloride dioxide is the inorganic compound with the formula MoO2Cl2. It is a yellow diamagnetic solid that is used as a precursor to other molybdenum compounds. Molybdenum dichloride dioxide is one of several oxychlorides of molybdenum.[1]
Structure
Gaseous molybdenum dichloride dioxide is a monomer,[2] but upon condensation, it polymerizes to give a coordination polymer of uncertain structure.
Preparation
MoO2Cl2 can also be prepared from MoOCl4:[3]
- MoOCl4 + O(Si(CH3)3)2 → MoO2Cl2 + 2 ClSi(CH3)3
It is also prepared by chlorination of molybdenum dioxide and molybdenum trioxide:[4][5]
- MoO2 + Cl2 → MoO2Cl2
- MoO3 + Cl2 → MoO2Cl2
Reactions
Many bisadducts are known of the type MoO2Cl2(ether)2. These octahedral molecular complexes are soluble in organic solvents. The adduct with dimethylsulfoxide can be readily prepared by treatment molybdenum trioxide with concentrated hydrochloric acid:[6]
- MoO3 + 2 HCl + 2 L → MoO2Cl2L2 + H2O
When treated with bulky anilines, MoO2Cl2 converts to diimido complexes, one of which is MoCl2(=N−Ar)2(dimethoxyethane). This complex is the precursor to the Schrock carbenes of the type Mo(OR)2(=N−Ar)(=CH−tBu).[4]
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1022. doi:10.1016/C2009-0-30414-6. ISBN 978-0-08-037941-8.
- ^ Ward, Brian G.; Stafford, Fred E. (1968). "Synthesis and Structure of Four- and Five-Coordinated Gaseous Oxohalides of Molybdenum(VI) and Tungsten(VI)". Inorganic Chemistry. 7 (12): 2569–2573. doi:10.1021/ic50070a020.
- ^ Gibson, V. C.; Kee, T. P.; Shaw, A. (1988). "New, Improved Synthesis of the Group 6 Oxyhalides, W(O)Cl4, W(O)2Cl2 and Mo(O)2Cl2". Polyhedron. 7 (7): 579–80. doi:10.1016/S0277-5387(00)86336-6.
- ^ a b Schrock, R. R.; Murdzek, J. S.; Bazan, G. C.; Robbins, J.; DiMare, M.; O'Regan, M. (1990). "Synthesis of molybdenum imido alkylidene complexes and some Reactions Involving Acyclic Olefins". Journal of the American Chemical Society. 112 (10): 3875–3886. Bibcode:1990JAChS.112.3875S. doi:10.1021/ja00166a023.
- ^ Takahashi, Hideyuki (14 January 2021). "Method of producing high bulk density molybdenum oxychloride".
- ^ Francisco J. Arnaiz (1997). "Dichlorodioxobis(Dimethylsulphoxide)-Molybdenum(VI)". Dichlorodioxobis(Dimethylsulphoxide) Molybdenum(VI). Inorganic Syntheses. Vol. 31. pp. 246–7. doi:10.1002/9780470132623.ch39. ISBN 978-0-470-13262-3.