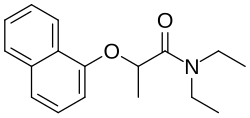
Napropamide
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Diethyl-2-naphthalen-1-yloxypropanamide | |
| Identifiers | |
3D model (JSmol)
|
|
| 2217870 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.035.742 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H21NO2 | |
| Molar mass | 271.360 g·mol−1 |
| Density | 1.18[1] |
| Melting point | 74.5 °C (166.1 °F; 347.6 K)[1] |
| Boiling point | 77.4 °C (171.3 °F; 350.5 K)[1] |
| 63 mg/L[1] | |
| Vapor pressure | 0.167 mPa[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
Napropamide is an acetamide herbicide. It was first sold under the trade name Devrinol,[2] and was first manufactured in 1969.[3] It is widely used in the European Union,[1] and in Australia.[4]
"Devrinol 50" is a wettable powder containing 50% napropamide.[5]
Napropamide's mode of action is unknown, therefore the HRAC classification system calls it equivalently Group Z or Group 0, although it was formerly classified as Group K (Group K3 or 15).[6]
Chemistry
Napropamide inhibits root growth. It is used against annual grasses and broadleaf weeds.[2] The d-isomer is noted as being significantly more effective than the racemic mixture against certain weeds.[3] Its formula is C17H21NO2.[2]
Tradenames
Napropamide has been sold as "Devrinol", "Jouster" and "Naprop".[5][1]
References
- ^ a b c d e f g Lewis KA, Tzilivakis J, Warner DJ, Green A (18 May 2016). "An international database for pesticide risk assessments and management". Human and Ecological Risk Assessment: An International Journal. 22 (4): 1050–1064. doi:10.1080/10807039.2015.1133242.
- ^ a b c Neal J (November 18, 2014). "Devrinol (napropamide)". NC State Extension. Retrieved November 26, 2024.
- ^ a b Wendeborn S, Godineau E, Mondière R, Smejkal T, Smits H (2012), "1.8 Chirality in Agrochemicals", Comprehensive Chirality, Elsevier, pp. 120–166, doi:10.1016/b978-0-08-095167-6.00102-6, ISBN 978-0-08-095168-3, retrieved 2024-11-26
- ^ "Devrinol 500WG Napropamide".
- ^ a b Williams H (1974). "Effects of certain preemergence herbicides on Diochondra spp". Proceedings of the Second International Turfgrass Research Conference: 410–417. doi:10.2135/1974.proc2ndintlturfgrass.c60.
- ^ "Australia Herbicide Classification Lookup". Herbicide Resistance Action Committee.
External links
- Napropamide in the Pesticide Properties DataBase (PPDB)
- Napropamide in the Pesticide Properties DataBase (PPDB) (Napropamide-M)