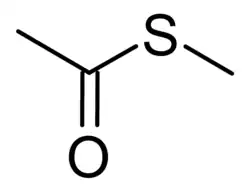
S-Methyl thioacetate
 | |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.775 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6OS | |
| Molar mass | 90.14 g·mol−1 |
| Density | 1.013 g/cm3 |
| Melting point | 96–97 °C (205–207 °F; 369–370 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
| |
S-Methyl thioacetate is the organosulfur compound with the formula CH3C(O)SCH3. This colorless, malodorous liquid is found in many plant species. In its pure form it has an unpleasant sulfurous smell, but when highly diluted and along with other simple alkyl thioacetates and related compounds, it is an important component of the odor and flavour profile of some foods, including Camembert cheese,[1][2] yogurt,[3] and baijiu.[4]
Alternate isomer
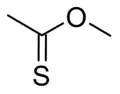
The constitutional isomer in which the oxygen and sulfur atoms are interchanged, O-methyl thioacetate, is also known as methyl thioacetate. It is not found in nature. It has mainly been the subject of theoretical studies.[6]
See also
References
- ^ Sablé S, Cottenceau G (December 1999). "Current knowledge of soft cheeses flavor and related compounds". Journal of Agricultural and Food Chemistry. 47 (12): 4825–36. Bibcode:1999JAFC...47.4825S. doi:10.1021/jf990414f. PMID 10606538.
- ^ Martínez-Cuesta Mdel C, Peláez C, Requena T (2013). "Methionine metabolism: major pathways and enzymes involved and strategies for control and diversification of volatile sulfur compounds in cheese". Critical Reviews in Food Science and Nutrition. 53 (4): 366–85. doi:10.1080/10408398.2010.536918. PMID 23320908. S2CID 27793531.
- ^ Ott, Andreas; Fay, Laurent B.; Chaintreau, Alain (1997). "Determination and Origin of the Aroma Impact Compounds of Yogurt Flavor". Journal of Agricultural and Food Chemistry. 45 (3): 850–858. Bibcode:1997JAFC...45..850O. doi:10.1021/jf960508e.
- ^ Liu, Huilin; Sun, Baoguo (2018). "Effect of Fermentation Processing on the Flavor of Baijiu". Journal of Agricultural and Food Chemistry. 66 (22): 5425–5432. Bibcode:2018JAFC...66.5425L. doi:10.1021/acs.jafc.8b00692. PMID 29751730.
- ^ "Methyl thioacetate". PubChem. U.S. National Library of Medicine.
- ^ Abboud, J. L. M.; Mo, O.; De Paz, J. L. G.; Yanez, M.; Esseffar, M.; Bouab, W.; El-Mouhtadi, M.; Mokhlisse, R.; Ballesteros, E. (1993). "Thiocarbonyl versus carbonyl compounds: A comparison of intrinsic reactivities". Journal of the American Chemical Society. 115 (26): 12468–12476. Bibcode:1993JAChS.11512468A. doi:10.1021/ja00079a030.