Cliropamine
 | |
| Clinical data | |
|---|---|
| Other names | Clipoxamine; D-16427; D-16,427 |
| Drug class | Sympathomimetic; Positive inotrope |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H25NO2 |
| Molar mass | 299.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
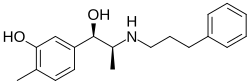
Cliropamine (INNTooltip International Nonproprietary Name; developmental code name D-16427) is a positive inotropic drug of the phenethylamine and amphetamine families which was never marketed.[1][2][3][4] It was first described by 1986.[4]
References
- ^ Negwer M, Scharnow HG (2001). Organic-chemical Drugs and Their Synonyms: An International Survey. Wiley-VCH. p. 1802. ISBN 978-3-527-30247-5. Retrieved 27 February 2025.
Cliropamine hydrochlo- ride ** , D - 16427 U Cardiotonic ( positive inotropic ) 8879 ( 7264 ) C19H25NO2 CH, 2- [Propyl(2-thienylethyl)amino]-5-hydroxytetralin-5,6,7,8-Tetrahydro-6-[propyl[2-(2-thienyl) ethyl] [...]
- ^ "CLIROPAMINE". Inxight Drugs. Retrieved 27 February 2025.
- ^ "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances" (PDF). World Health Organization. Geneva. 2004.
- ^ a b Engel J, Bickel E, Klingler KH, Schönenberger H (December 1986). "[14C-labeling of D 16,427, a new positive inotropic agent]". Arch Pharm (Weinheim) (in German). 319 (12): 1113–1116. doi:10.1002/ardp.19863191210. PMID 2882738.
| Phenethylamines |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphetamines |
| ||||||||||||||||
| Phentermines |
| ||||||||||||||||
| Cathinones |
| ||||||||||||||||
| Phenylisobutylamines (and further-extended) | |||||||||||||||||
| Catecholamines (and close relatives) |
| ||||||||||||||||
| Cyclized phenethylamines |
| ||||||||||||||||
| Related compounds |
| ||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under Creative Commons Attribution-Share Alike 4.0 unless otherwise noted. Additional terms may apply for the media files.